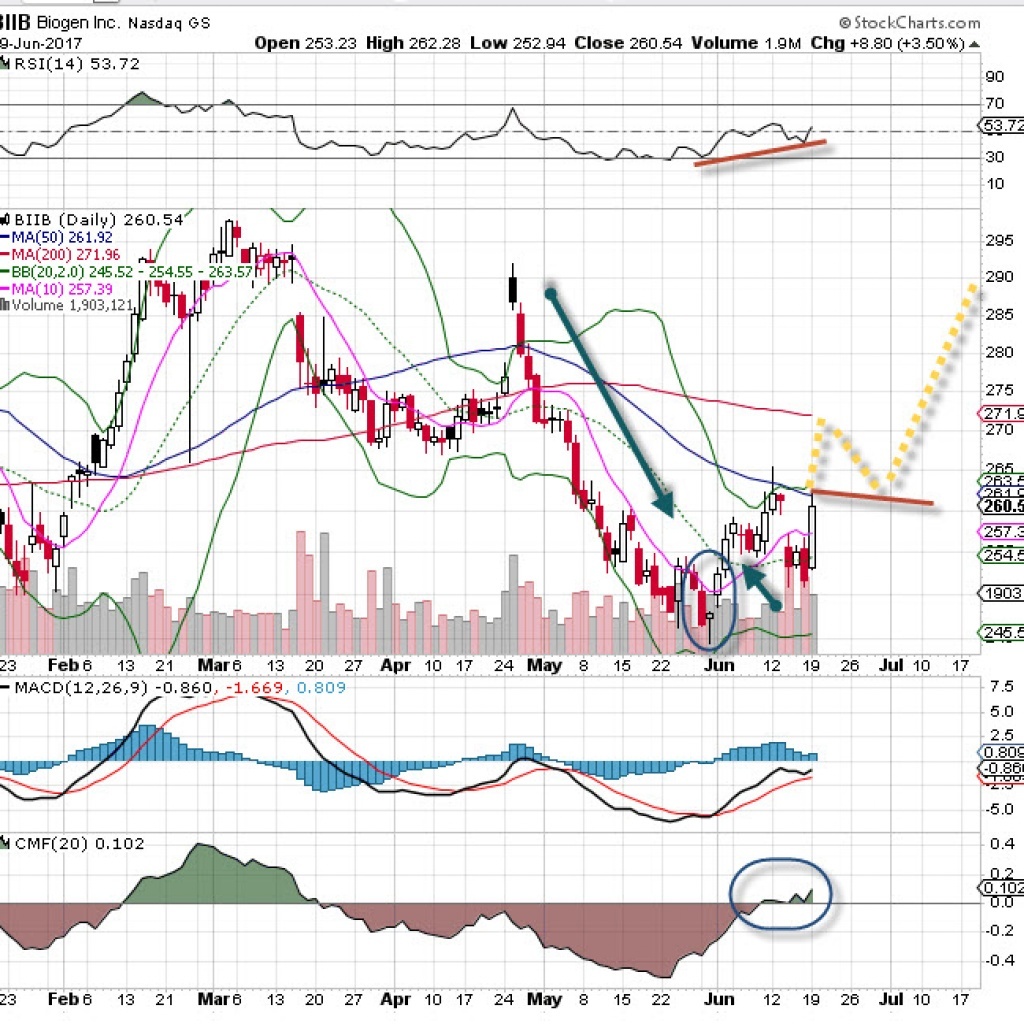
The big biopharma company Biogen seems poised for an upside pop soon based on recent turnover and bullish price divergence. Back in April, the stock took a major tumble of more than 20% on heavy turnover. It now appears to have bottomed out. It made a nice morningstar pattern (circled) and a higher low on the chart. What I really find intriguing is the bullish turn on money flow (bottom pane). In April that was not a positive sign, so this is an important divergence.
The bullish candle on June 19 filled the nasty gap down from the prior week. At this point, Biogen has a good chance to reach the 200 ma for the first time since breaking down in late April. I think it is likely to follow the yellow dotted lines. MACD has also held positive, so we are bullish on this name to at least reach the breakdown level in April.
Biogen (Nasdaq: BIIB) Video Chart Analysis
Take a deeper dive into the chart action on tech stock Intercontinental Exchange Nasdaq:BIIB and learn how to read the technicals. Get Bob Lang’s full analysis as he marks up our chart of the week.
Love what you’re learning in our market analysis? Don’t miss a single video! Get the latest chart action delivered directly to your inbox every week as Bob breaks down stocks to watch and potential trade options!
About Biogen
Biogen Inc., a biopharmaceutical company, discovers, develops, manufactures, and delivers therapies for the treatment of neurological and autoimmune diseases worldwide. The company offers TECFIDERA, AVONEX, PLEGRIDY, TYSABRI, ZINBRYTA, and FAMPYRA to treat multiple sclerosis (MS); FUMADERM for the treatment of plaque psoriasis; and SPINRAZA to treat spinal muscular atrophy. It also provides BENEPALI, an etanercept biosimilar referencing ENBREL; FLIXABI, an infliximab biosimilar referencing REMICADE; RITUXAN for the treatment of non-Hodgkin’s lymphoma, chronic lymphocytic leukemia (CLL), and other conditions; GAZYVA to treat CLL and follicular lymphoma; and other potential anti-CD20 therapies. The companys Phase III clinical trial products comprise GAZYVA for the treatment of front-line indolent non-hodgkins lymphoma; and Aducanumab and E2609 for Alzheimers disease. Its Phase II clinical trial products include BIIB074 for trigeminal neuralgia, lumbosacral radiculopathy, and erythromelalgia; BAN2401 for Alzheimer’s disease; Opicinumab (anti-LINGO-1) for MS; TYSABRI for acute ischemic stroke